Transcranial Magnetic Stimulation (TMS): Innovation in the Treatment of Depression
What is NeuroStar TMS?
TMS treatment stimulates nerve cells in an area of the brain believed to be linked to depression. A treatment coil is applied to the patient’s head through which the NeuroStar TMS therapy system generates highly concentrated magnetic field pulses that turn on and off rapidly. The pulses continue for the duration of the session. Also, it’s a safe, effective, non-drug depression treatment.
Does it hurt?
Minor discomfort at treatment site (where the device touches your head) that subsides within the first week of treatment. There is no sedation or impact on your alertness. You can read, watch TV, or talk with your TMS coordinator during your session and can drive home immediately after treatment. Each TMS treatment session is a 30-minute outpatient procedure that is prescribed by a psychiatrist, does not require anesthesia or sedation, and patients remain awake and alert. The treatment is typically administered daily for 7 to 8 weeks and the patient is able to maintain their normal routine before and after beginning TMS therapy.
Does it work?
“A real-world study reported an 83% response rate. This means that 83% of patients that completed their NeuroStar treatment cycle saw measurable improvement in their depression symptoms.**,12 The same real-world study reported a 62% remission rate. This means that 62% of patients that completed their NeuroStar treatment cycle likely wouldn’t be diagnosed with MDD if they were first being evaluated by their doctor.**,12” https://neurostar.com/is-neurostar-safe-proven/
How much does it cost?
Over 300 million people have insurance plans that cover NeuroStar, including Medicare and Tricare. Conditions and coverage can vary by insurance company, and your dedicated NeuroStar coordinator will help you determine your benefits and coverage.
Nationally Recognized Leader in TMS Therapy
What sets us apart from other Transcranial Magnetic Stimulation providers is our expertise. Lindner Center of HOPE is nationally recognized as a leader in the treatment of depression. Our reputation is a result of years of clinical research and experience in mental illness and collaboration with academic centers such as Johns Hopkins and the Mayo Clinic through the National Network of Depression Centers. Our own ongoing research and collaborative efforts enable the Center to harness the most current data.
For patients, this means the best of the best – the best clinical minds, the best data and the best technology are being applied to achieve successful outcomes.
Call (513) 536-0864 to discuss TMS therapy.
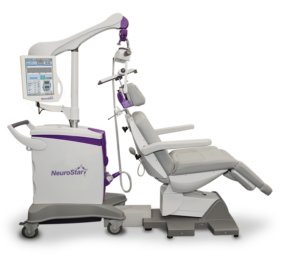
TMS treatment is conducted using a device called the NeuroStar TMS Therapy System in the state-of-the-art Farmer Family Neuromodulation Center. While treatment is administered, patients remain awake while sitting in a comfortable reclining chair.
Each TMS treatment session is a 30-minute outpatient procedure that is prescribed by a psychiatrist, does not require anesthesia or sedation, and patients remain awake and alert. The treatment is typically administered daily for 7 to 8 weeks and the patient is able to maintain their normal routine before and after beginning TMS therapy.
TMS treatment stimulates nerve cells in an area of the brain believed to be linked to depression. A treatment coil is applied to the patient’s head through which the NeuroStar TMS therapy system generates highly concentrated magnetic field pulses that turn on and off rapidly. The pulses continue for the duration of the session.
Some Stats on TMS Efficacy
“A real-world study reported an 83% response rate. This means that 83% of patients that completed their NeuroStar treatment cycle saw measurable improvement in their depression symptoms.**,12 The same real-world study reported a 62% remission rate. This means that 62% of patients that completed their NeuroStar treatment cycle likely wouldn’t be diagnosed with MDD if they were first being evaluated by their doctor.**,12” https://neurostar.com/is-neurostar-safe-proven/
Now Offering Touchstar (Thetaburst) TMS
- 5 minute procedure, 600 pulses total
- Uses higher frequency pulses to ensure effective dosage is being administered every treatment in a shorter amount of time.
- 36 treatments for 7-8 weeks
- Best for those who may not have time for 30-minute treatments, anyone who is unable to sit comfortably for 30 minutes, or anyone who is unable to tolerate sensation of other protocols
- Touchstar is a 3.5 minute intermittent thetaburst (iTBS) protocol
- FDA cleared in 2021
Now Offering TMS for Adolescent MDD
- 36 treatments, 30-minute sessions (essentially the same protocol as MDD for adults. Same contraindications and side effects)
- …with that being said, braces and retainers are not contraindications
- Adolescents ages 15-21
- We use PHQ-9, MADRS, and CGI scores to assess patient improvement
- Covered by some insurance companies (OOP is available)
- Requires referral from OP Provider (MD, DO, NP, etc.)
- Consultation with LCOH TMS certified provider prior to initial mapping
Now Offering TMS for OCD
- TMS is used as an adjunct for OCD therapy/medications
- Patient must actively be in treatment with an OP provider for OCD
- Must be willing to engage in ERP practices while in the TMS treatment
- We use personalized symptom provocation to increase efficacy of treatment
- 29 treatments, 30-minute sessions (M-F) for 5-6 weeks
- Rather than treating in the left dorsolateral prefrontal cortex as we would for depression, OCD is treated in the dorsomedial prefrontal cortex (top of the head)
- Same side-effects and contraindications as MDD
- Not currently covered by most insurances (OOP is available)
- Requires referral from OP Provider (MD, DO, NP, etc.) with follow-up clinical from OCD/ERP therapist or provider (if not the same provider as the referring provider)
- Consultation with LCOH TMS certified provider prior to initial mapping to determine if patient is appropriate
- FDA cleared for OCD in 2022
Benefits:
- Does not require anesthesia, non-invasive, well tolerated.
- An outpatient service – patient can continue normal daily routines.
- Current data demonstrates efficacy in patients who have struggled with medication.
- May be good alternative for patients who responded to ECT in past.
- No significant memory impairment.
- FDA Approved in 2008 for the treatment of depression.
Considerations:
- Possible side effects:
- Facial twitching during the treatment
- Skin redness at site of coil placement
- Anxiety before and during treatment
- Mild discomfort (usually dissipates by end of first treatment)
- Headache
- Expense – Often covered by third-party insurance. Private pay is also accepted.
- Time – requires 36 treatments of a half hour in length over 7 to 8 weeks
Ira Herman, a TMS patient of Lindner Center of Hope
 For the last 14 years, I have had recurring episodes of severe, totally disabling clinical depression, each lasting between 4 and 14 months. In recent years, the episodes were getting longer and closer together. It seemed to me that I was looking at spending the rest of my life in severe clinical depression.
For the last 14 years, I have had recurring episodes of severe, totally disabling clinical depression, each lasting between 4 and 14 months. In recent years, the episodes were getting longer and closer together. It seemed to me that I was looking at spending the rest of my life in severe clinical depression.
I have been through at least 6 psychiatrists, have tried all of the commonly prescribed meds. Nothing was helping me… until I found THE COMPLETE ANSWER (at least for me): Transcranial Magnetic Stimulation Therapy (TMS). On 2/2/10, I walked into Lindner Center of HOPE, in Mason Ohio. At that time, I was not able to drive safely and could barely find my way into the parking lot.
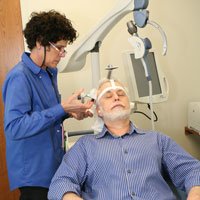
After a series of 32 TMS treatments, one-a-day for six-weeks, I am in complete remission. They gave me my life back. I personally consider this new treatment to be a miracle from God, and it’s a recent scientific medical breakthrough that, I predict, will revolutionize the treatment of “treatment-resistant” depression.
I am back to 100% normal and went from being 100% disabled to 100% able through these treatments. It works–at least it did for me, when NOTHING else did.
To determine eligibility for TMS therapy, patients begin by scheduling an initial assessment with a TMS prescribing psychiatrist. During this initial assessment, the psychiatrist will:
- Determine a diagnosis
- Evaluate appropriateness of TMS Therapy
- Determine the risks and benefits of TMS compared to other available treatments for your diagnosis.
The psychiatrist will want details about previous treatment for your depression including counseling history, names of medications, maximum dosage, duration of treatment and reasons treatment was discontinued such as lack of benefit or side effects.
A history and physical examination from your primary care physician may also needed prior to the first treatment, if indicated. At the end of the assessment the psychiatrist will decide if you are a candidate for TMS Therapy. If TMS Therapy is right for you, and you pursue treatment, your next appointment will last one hour. During this visit the psychiatrist will create a plan for your treatment.
Please answer yes or no to each of the following:
Have you been diagnosed with or are you being treated for depression?
Have you tried anti-depressant medication(s) and failed to achieve satisfactory improvement from your depression?
Are you in generally good health?
TMS Therapy uses magnetic pulses, so it is important that you do not have any implanted metallic devices or non-removable metallic objects in or around your head. Can you say that you do NOT have any of the following:
- Metal plates in your head
- Aneurysm coils
- Stents
- Bullet fragments or other metal devices in your head
- Pacemaker
- Implantable cardioverter defibrillators or vegus nerve stimulators.
TMS Therapy is covered by insurance in most cases.
If you answered yes to all of the questions, you may be a candidate for TMS Therapy. Ask your doctor or call 513 536 HOPE (513-536-0864) and ask about TMS Therapy.
Insurance policies and benefits vary greatly. Below is an overview of the payment process for TMS Therapy at Lindner Center of HOPE.
- The initial consultation with a psychiatrist to determine whether TMS Therapy is appropriate is typically covered by insurance. Lindner Center of HOPE will collect patient insurance information prior to your appointment and patient will be responsible to pay co-pay or appropriate charges at your consult appointment. Lindner Center of HOPE will then file the claim with your insurance company.
- TMS Therapy itself, approved by the US FDA in 2008, may not be covered by all major health plans.
- Lindner Center of HOPE Registration staff can inform patients of their TMS benefits.
- Payment for TMS Therapy is made by the patient at the time of service and payment guidelines will be outlined.
- If TMS Therapy patients would like to submit charges to their insurance company, Lindner Center of HOPE will provide patients claim information. In the meantime, payment for TMS Therapy is made by the patient at the time of service. Payment guidelines will be outlined by the counselor.
- Lindner Center of HOPE can provide patients information to obtain financing through outside services.
Financial Counselors
Lindner Center of HOPE Financial Counselors are available from 8am to 6pm Monday through Friday to assist you in applying for Financial Assistance. Call 513-536-0224
Frequently Asked Questions about Transcranial Magnetic Stimulation Therapy
Why TMS therapy?
Depression has been linked to abnormal function of nerve cells in a specific part of the brain. Highly focused magnetic field pulses used in Transcranial Magnetic Stimulation (TMS) therapy gently stimulate these nerve cells believed to be linked to depression. Evidence shows that TMS is effective in the treatment of moderate to severe depression in patients with a history of treatment resistant depression. It is also associated with fewer hospitalizations and doctor visits.
How does TMS therapy work?
TMS treatment is conducted using a device called the NeuroStar® TMS Therapy System.
While treatment is administered, patients remain awake while sitting in a comfortable reclining chair. A treatment coil is applied to the head and the NeuroStar® TMS Therapy System generates highly concentrated magnetic field pulses that turn on and off rapidly.
In clinical trials, patients reported relief from the emotional effects of depression and experienced improvement in anxiety, changes in appetite, body aches and lack of energy – all physical symptoms of depression.
How many TMS therapy sessions will I need?
Once approved by a psychiatrist for TMS, the patient will be scheduled for a treatment series consisting of five treatments per week (once every day except weekends) over a 7-8 week period for a total of 36 treatments. Each treatment lasts approximately 30 minutes.
What does treatment feel like?
During a TMS treatment, the patient will feel a tapping sensation on the scalp. TMS therapy produces a loud clicking sound, so earplugs are provided.
The most common adverse reaction related to treatment was scalp pain or discomfort at the treatment area during active treatments. This reaction was brief, mild to moderate in severity and declined markedly after the first week of treatment.
What should a patient expect when receiving TMS therapy?
Treatment does not involve any anesthesia or sedation and patients remain awake and alert during the treatment. Treatment takes place in an inviting environment with options to rest, listen to meditations, podcasts, music, watch appropriate shows/movies and talk to staff. Patients are able to drive and return to work or other daily activities immediately after treatment.
What should a patient expect after a TMS treatment?
Since TMS treatment does not involve any anesthesia or sedation. During a treatment session patients remain awake and alert during the treatment. Patients are able to drive and/or return to work and/or other daily activities immediately after treatment.
What are the TMS therapy benefits?
In an open-label clinical trial (resembling real world practice) approximately 1 in 2 patients treated with TMS therapy experienced a significant improvement in depression symptoms, and 1 in 3 experienced complete resolution.
- Does not require anesthesia.
- Non invasive.
- Well tolerated.
- Can be done entirely as an outpatient and can drive self to and from the treatment as well as continue normal daily routines, including work, even on treatment days.
- Current data has demonstrated efficacy in patients who have struggled with medication efficacy for their depression.
- May be good alternative for patients who responded to ECT in past.
- No significant memory impairment.
We use PHQ-9, MADRS, and CGI scores to assess patient improvement.
PHQ-10 Self-Assessment Health Questionnaire
How soon would benefits of TMS treatment be apparent?
Most patients have experienced results by the fourth week of treatment, however everyone is different and some patients may notice benefits over a shorter or longer period of time. During your treatment, a psychiatrist will consult with you to evaluate your progress.
What are the side effects of TMS therapy?
The most commonly reported side effect was headache or scalp pain and these side effects were generally mild to moderate and occurred less frequently after the first week of treatment.
What is the difference between TMS therapy & Antidepressants?
TMS Therapy is not a medication so it does not circulate in the blood stream. It does not produce side effects such as weight gain, sexual dysfunction, nausea, dry mouth, and sedation that are often associated with anti¬depressants. The most common side effects reported during clinical trials were headache and scalp pain or discomfort – generally mild to moderate – occurring less frequently after the first week of treatment. In some instances the side effects are so severe that the patient discontinues treatment.
What is the difference between TMS therapy and ECT?
TMS therapy and ECT are both procedures that treat major depression. The important differences between TMS therapy and ECT include:
- ECT requires anesthesia whereas TMS therapy does not
- ECT uses applies electrical energy whole brain, while TMS uses magnetic energy applied only to the left prefrontal cortex.
- TMS therapy is a non-invasive procedure that does not require anesthesia and does not produce a seizure.
With any treatment, patients and clinicians should work together to determine the most appropriate option.
Does TMS therapy cause memory loss?
TMS Therapy was systematically evaluated for its effects on memory. Clinical trials demonstrated that Neurostar® TMS Therapy does not result in adverse effects on memory or concentration.
Who should NOT have TMS therapy?
TMS Therapy should not be administered to persons who have non-removable magnetic-sensitive metal in their head or other part of their body that would be within 12 inches of the NeuroStar® Magnetic Coil. Objects that may have this kind of metal include:
- Aneurysm clips or coils
- Stents
- Implanted Stimulators
- Electrodes to monitor brain activity
- Ferromagnetic implants in your eyes or ears
- Bullet fragments and other metal devices or objects implanted in the head
It should also not be used by patients with implanted devices that are controlled by physiological signals such as pacemakers, etc. If you have questions about metal or implanted devices in your body, please consult a Lindner Center of HOPE psychiatrist.