Category: TMS
Transcranial Magnetic Stimulation (TMS) is an innovative treatment for depression. Depression is common and affects 1 in 10 adults. Only a fraction of individuals suffering from depression seek treatment. Of those who do, greater than 30% fail to achieve satisfactory improvement. Not all patients improve when treated with medications or psychotherapy. Up to 25% of people suffering from depression will not respond to multiple trials of medication due to a lack of efficacy or difficulty tolerating medication. Likewise, many people struggle to respond to the best efforts of psychotherapy, either due to a lack of response or a lack of time and/or financial resources that are necessary for psychotherapy interventions. Alternate treatment modalities are critical to addressing the ongoing needs of patients who suffer from the debilitating effects of depression.
Understanding the Benefits of Transcranial Magnetic Stimulation
Evidence shows that TMS is effective in the treatment of moderate to severe depression in patients with a history of treatment resistance. Depression has been linked to an abnormal function of nerve cells in a specific part of the brain. Highly focused magnetic field pulses used in Transcranial Magnetic Stimulation (TMS) therapy gently stimulates these nerve cells. New data emerging from recent studies suggests that in most patients, the clinical benefits of TMS therapy are maintained through 12 months.
How Transcranial Magnetic Stimulation Works
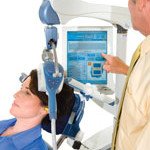
TMS is a non-invasive, localized treatment conducted using a device that delivers rapidly pulsating and localized magnetic fields that activate a subset of nerve cells in the front part of the brain.
While treatment is administered patients remain awake while sitting in a comfortable reclining chair. A treatment coil is applied to the head and the system generates highly concentrated magnetic field pulses. Transcranial Magnetic Stimulation is delivered in a series of 37-minute outpatient treatments, typically administered daily, (5 days per week) for 4 to 6 weeks. Technological advancements in equipment has led to decreased treatment durations.
Pros and Cons of TMS Therapy
Some advantages and disadvantages of TMS include:
- It does not require anesthesia
- Non-invasive
- Well tolerated
- An outpatient service and patient continues normal daily routines
- Current data demonstrates efficacy in patients who have struggled with medication
- May be good alternative for patients who responded to Electroconvulsive Therapy (ECT) in the past
- No significant memory impairment
- FDA Approved in 2008 for the treatment of depression
Cons of TMS Therapy
- Facial twitching during the treatment
- Skin redness at site of coil placement
- Anxiety before and during treatment
- Mild discomfort (usually dissipates by end of first treatment)
- Headache
- Process for insurance coverage can be cumbersome
- Time required 30 treatments over 6 weeks
TMS at Lindner Center of HOPE
The Lindner Center of HOPE is a nationally recognized Leader in TMS Therapy. Our expert reputation is a result of years of clinical research and experience in mental illness and collaboration with academic centers such as Johns Hopkins and the Mayo Clinic through the National Network of Depression Centers. For patients, this means the best of the best – the best clinical minds, the best data and the best technology are being applied to achieve successful outcomes.
There is HOPE. For more information on TMS Therapy, call (513) 536-4674 or click here.
